Rapid technological advances in microfluidics, imaging and digital geneexpression profiling are converging to present new capabilities for blood, tissue and single-cell analysis. Our laboratory is interested in taking these advances and creating new technologies to help build understanding of the metastatic process. Our research focus is on:
- The development and application of microfluidic devices and biomaterials for the isolation and characterization of extracellular vesicles
- The enrichment and analysis of CTCs at a single cell level
- Novel imaging strategies to characterize tumor tissue, cancer cells, and extracellular vesicles
Extracellular Vesicle Isolation and Characterization
Extracellular vesicles (EVs), such as exosomes, microvesicles, and oncosomes, are small particles that bud off of cancer cells, with some cancer cells releasing up to thousands of EVs per day. Researchers have hypothesized that these EVs shed from tumors transport RNA, DNA and proteins that promote tumor growth, and studies have shown that EVs are present in the blood of most cancer patients. Ongoing work in my lab incorporates microfluidics and novel biomaterials to enrich cell-specific EVs from cancer patients, using as little as 1mL of plasma. Once isolated, we are exploring their protein and nucleic acid content to probe their potential as a less invasive biomarker. Dropletbased microfluidics are being developed to probe the EVs at a single vesicle level.
Microfluidics for Circulating Tumor Cell Analysis
One of the proposed mechanisms of cancer metastasis is the dissemination of tumor cells from the primary organ into the blood stream. A cellular link between the primary malignant tumor and the peripheral metastases has been established in the form of CTCs in peripheral blood. While extremely rare, these cells provide a potentially accessible source for early detection, characterization and monitoring of cancers that would otherwise require invasive serial biopsies. Working in collaboration with Drs. Mehmet Toner, Shyamala Maheswaran and Daniel Haber, we have designed a high throughput microfluidic device, the CTC-Chip, which allows the isolation and characterization of CTCs from the peripheral blood of cancer patients. Using blood from patients with metastatic and localized cancer, we have demonstrated the ability to isolate, enumerate and molecularly characterize putative CTCs with high sensitivity and specificity. Ongoing projects include translating the technology for early cancer detection, exploring the biophysics of the CTC clusters, and the design of biomaterials for the gentle release of the rare cells from the device surface. We are also developing new strategies for the long term preservation of whole blood such that samples can be shipped around the world for CTC analysis.
High-Content and High-Throughput Imaging of Tumor Specimens
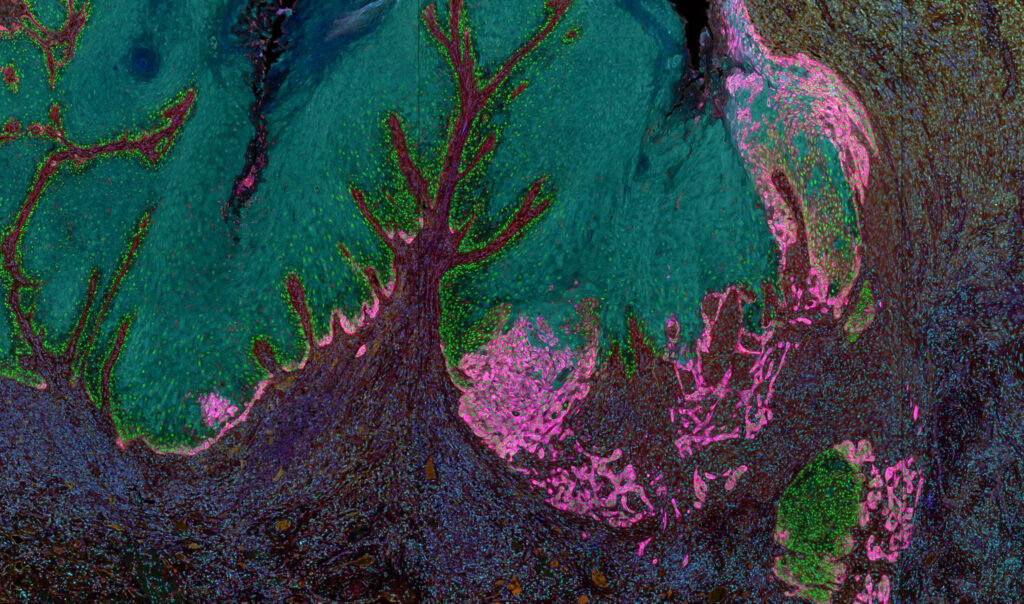
Tumors can be highly heterogeneous, and their surrounding stroma even more so. Traditionally, the tumor and surrounding cells are dissociated from the tissue matrix for high throughput analysis of each cell. While this allows for important information to be gained, the spatial architecture of the tissue and corresponding interplay between tumor and immune cells can be lost. The Stott lab is developing quantitative, robust analysis for individual cells within the tumor and neighboring tissue using multispectral imaging. We are using this technology alongside downstream imaging processing algorithms to interrogate signaling activity in cancer cells, immune cell infiltration into to the tumor and pEMT in cancer cells. These data will be used to gain an increased understanding in the relationship between pharmacologic measurements and clinical outcomes, ultimately leading to the optimization of patient therapy.
